Patterns in electron configuration worksheet answer key
Table of Contents
Table of Contents
Have you ever struggled with drawing electron dot structures? This fundamental concept in chemistry can be confusing and overwhelming, but fear not! In this article, we will break down the process step-by-step and provide helpful tips along the way. By the end, you’ll be able to confidently draw electron dot structures for any molecule.
Many students struggle with drawing electron dot structures because they don’t understand the importance of valence electrons. These are the outermost electrons in an atom, and they are responsible for bonding with other atoms to form molecules. Without a clear understanding of valence electrons, drawing electron dot structures can be a frustrating experience.
The first step in drawing electron dot structures is to determine the number of valence electrons for each atom in the molecule. This can be done by referring to the periodic table and looking at the element’s group number. For example, atoms in group 1A have 1 valence electron, group 2A have 2 valence electrons, and so on.
Once you have determined the number of valence electrons for each atom, you can start drawing the structure. The basic idea is to place dots around each atom to represent its valence electrons. Each dot represents one electron, and the dots are placed around the symbol for the atom. When atoms bond together, they share electrons, and this is represented by a line between the atoms.
In summary, drawing electron dot structures involves determining the number of valence electrons for each atom in the molecule, placing dots around each atom to represent its valence electrons, and connecting the atoms with lines to represent the shared electrons. Practice makes perfect, so keep practicing and soon you’ll be a pro at drawing electron dot structures!
My Experience with Drawing Electron Dot Structures
When I was first learning about electron dot structures, I found it difficult to keep track of the valence electrons for each atom in a molecule. However, after practicing with several examples and referring to the periodic table, it started to become easier. One helpful tip I learned is to draw the structure multiple times, each time focusing on a different portion of the molecule. This helped me to better understand how the electrons were shared between atoms.
Tips for Drawing Electron Dot Structures
One helpful tip when drawing electron dot structures is to start with the atoms that have the highest number of valence electrons. These atoms are typically the central atoms in a molecule, and they are surrounded by the other atoms in the structure. Another tip is to pay close attention to the octet rule, which states that atoms tend to bond in a way that gives them a full outer shell of 8 electrons. Finally, practice makes perfect! Keep practicing with different molecules until you feel comfortable with the process.
The Importance of Electron Dot Structures in Chemistry
Electron dot structures are an important concept in chemistry because they help us to understand how molecules are formed and how they interact with each other. By understanding the electron configuration of atoms and the way that they bond with each other, we can predict the properties of different compounds and how they will react in various chemical reactions.
Common Mistakes When Drawing Electron Dot Structures
One common mistake when drawing electron dot structures is forgetting to place dots around each individual atom. Another mistake is not paying attention to the charge of individual atoms or the molecule as a whole. This can lead to incorrect structures and a misunderstanding of the molecule’s properties. It’s important to always double-check your work and refer back to the periodic table as needed.
Practice Makes Perfect
As with any new concept in chemistry, practice makes perfect when it comes to drawing electron dot structures. Start with simple molecules and work your way up to more complex ones. Challenge yourself by attempting to draw the structure of a molecule you’ve never seen before. Before you know it, you’ll be drawing electron dot structures like a pro!
Question and Answer
Q: Are there any shortcuts to drawing electron dot structures?
A: While there are no shortcuts to drawing electron dot structures, there are some helpful tips that can make the process easier. For example, paying attention to the octet rule and starting with the central atom can save time and make the structure more efficient.
Q: Can you draw electron dot structures for any molecule?
A: Yes, electron dot structures can be drawn for any molecule as long as you know the number of valence electrons for each atom in the molecule.
Q: How can electron dot structures be used to predict the properties of a molecule?
A: Electron dot structures can be used to predict the geometry and polarity of a molecule, as well as its reactivity in chemical reactions. By understanding the way that atoms bond with each other in a molecule, we can better understand its properties and behavior.
Q: What is the purpose of drawing electron dot structures?
A: The purpose of drawing electron dot structures is to represent the valence electrons and bonding in a molecule. This helps us to better understand the way that atoms interact with each other to form a compound, and to predict its properties and behavior in different chemical reactions.
Conclusion of How to Draw Electron Dot Structures
Understanding how to draw electron dot structures is an essential skill in chemistry, and can open doors to a deeper understanding of the properties and behavior of different molecules. By following the steps outlined in this article, you can confidently draw electron dot structures for any molecule and use them to predict the molecule’s properties and behavior in chemical reactions.
Gallery
PPT - #7 Draw Electron Dot Structures For Each Molecule PowerPoint
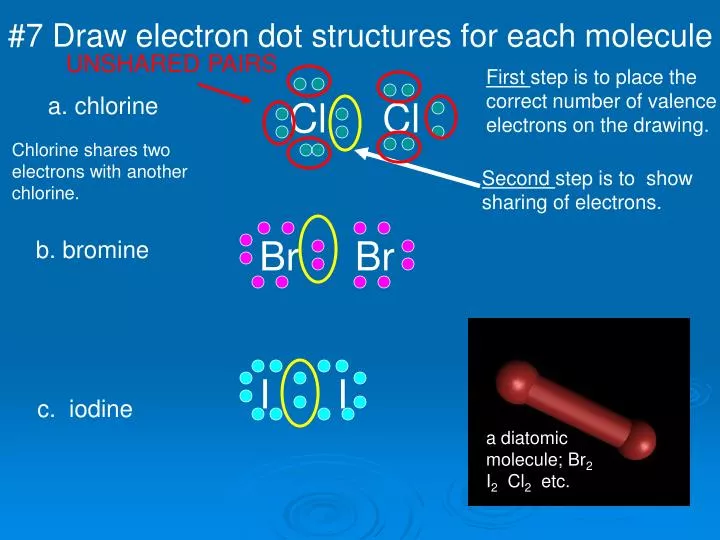
Photo Credit by: bing.com / electron dot draw ppt molecule structures each chlorine presentation powerpoint drawing slide1
Molecular Modeling – Digital And Analog | Middlebury College Chem 103 Lab

Photo Credit by: bing.com / molecular science beaker compounds covalent middlebury electron bonding struktur valence electrons ionic chem chemische verbindingen kidsworksheetfun periodic chemie 2592 filmpje
Multimedia: Represent Bonding With Lewis Dot Diagrams | Chapter 4

Photo Credit by: bing.com / lewis dot diagrams table hydrogen elements periodic structures calcium multimedia middle school showing lesson chapter chemistry
Draw The Electron Dot Structure Of F2. Chemistry - Q&A

Photo Credit by: bing.com / electron ethanoic propanone
Patterns In Electron Configuration Worksheet Answer Key - Lewis Dot

Photo Credit by: bing.com / diagrams configuration rubidium configurations